BUTRANS PATCHES BUPRENORPHINE 10MG 4PATCH
$150.00 Original price was: $150.00.$135.00Current price is: $135.00.
- Premium Quality
- Secure Payments
- Satisfaction Guarantee
- Worldwide Shipping
- Money Back Guarantee
| LABORATORY: | GRÜNENTHAL |
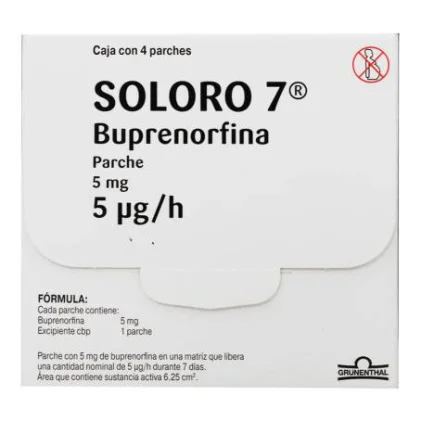
| BRAND NAME: | BUTRANS (SOLORO 7 IN MEXICO) |
| ACTIVE INGREDIENT: | BUPRENORPHINE 5MG |
| CONTAINT: | 4 PATCHES |
SOLORO® 7 is indicated for the treatment of moderate to severe chronic pain. SOLORO® 7 5 μg / h: Each PATCH contains: Buprenorphine 5 mg Excipient cbp 1 patch. Patch with 10 mg of buprenorphine in a matrix that releases a nominal amount of 5 μg / h for 7 days. Area containing active substance 12.5 cm2.
Description
Pharmacodynamic properties: Buprenorphine is a partial μ-opioid agonist. It also has antagonistic activity at the kappa-opioid receptor. It is classified as a psychotropic substance under international convention.
Opioids can influence the hypothalamic-pituitary-adrenal or –gonadal axes. Some of the changes that can be observed include an increase in serum prolactin and decreases in plasma levels of cortisol and testosterone. Clinical symptoms may manifest due to these hormonal changes.
In vitro and animal studies indicate different effects of natural opiates, such as morphine, on components of the immune system; The clinical significance of these findings is unknown. It is unknown whether buprenorphine, a semi-synthetic opiate, has immunological effects similar to morphine.
Each SOLORO® 7 buprenorphine transdermal patch provides a constant supply of buprenorphine for up to 7 days. Steady state is reached during the first application on the third day. After removing the patch, buprenorphine concentrations decrease, reducing by approximately 50% in 12 hours (range 10-24 hours).
Absorption does not vary significantly between specified application sites. The mean exposure (AUC) at each of the application sites is within approximately +/- 11% of the mean exposure for all four sites.
After application of the buprenorphine transdermal patch, the active substance diffuses from the system through the skin.
Buprenorphine is approximately 96% bound to plasma proteins.
The metabolism of buprenorphine in the skin after patch application is negligible. Buprenorphine is eliminated via hepatic metabolism with subsequent biliary and renal excretion of soluble metabolites.
Norbuprenorphine is the only known active metabolite of buprenorphine
Be the first to review “BUTRANS PATCHES BUPRENORPHINE 10MG 4PATCH” Cancel reply
Related products
Pain Relief
Pain Relief
Pain Relief
Pain Relief
Pain Relief
Pain Relief
Pain Relief





Reviews
There are no reviews yet.